Read our article on Delayed Cord Clamping & the Vitamin K Shot to understand why newborns do not naturally receive significant Vitamin K at birth.
PHYTONADIONE#
Phytonadione (Vitamin K₁) is a synthetic, fat-soluble vitamin administered to newborns within hours of birth and used in adults to reverse anticoagulant effects—most commonly those caused by vitamin K antagonists such as warfarin—or to treat vitamin K deficiency. While presented as essential for blood clotting factor production, the injection contains benzyl alcohol preservative (9 mg/mL in multidose formulations), a known neurotoxin associated with “gasping syndrome” and fatal reactions in neonates. The FDA mandates a BLACK BOX WARNING for severe hypersensitivity reactions including anaphylaxis and death following intravenous and intramuscular administration. Despite being given to virtually all newborns, vitamin K deficiency bleeding is extremely rare (1.7 per 100,000 births), and the injection bypasses natural oral vitamin K absorption, delivering supraphysiologic doses with toxic preservatives directly into tissue and bloodstream of hours-old infants with immature detoxification systems.
Mechanism of Action#
What the Mechanism Affects#
Brand Names#
Prescribed For#
- Anticoagulant-Induced Hypoprothrombinemia: Correction of excessive INR prolongation caused by warfarin, coumarin, or indanedione derivatives in adults requiring urgent reversal for bleeding or emergency surgery.
- Vitamin K Deficiency Bleeding (VKDB) Prophylaxis in Newborns: Universal intramuscular injection (0.5-1 mg) within 1 hour of birth to prevent rare early (0-24 hours), classical (1-7 days), and late (2-12 weeks) VKDB. Incidence without prophylaxis: 1.7 per 100,000 births.
- Treatment of Vitamin K Deficiency Bleeding in Neonates: Treatment dose of 1 mg subcutaneous or intramuscular for active bleeding in newborns. Higher doses if mother received anticoagulants during pregnancy.
- Hypoprothrombinemia Due to Malabsorption: In adults with obstructive jaundice, biliary fistula, celiac disease, ulcerative colitis, sprue, cystic fibrosis, regional enteritis, short bowel syndrome, or prolonged parenteral nutrition without vitamin K supplementation.
- Drug-Induced Vitamin K Deficiency: From antibiotics that suppress intestinal vitamin K-producing bacteria (especially cephalosporins, broad-spectrum penicillins), chronic salicylate use, or other drugs interfering with vitamin K metabolism.
Contraindications & Black Box Warning#
Severe reactions, including fatalities, have occurred during and immediately after
INTRAVENOUS injection of AquaMEPHYTON* (Phytonadione), even when precautions have been
taken to dilute the AquaMEPHYTON and to avoid rapid infusion. Severe reactions, including
fatalities, have also been reported following INTRAMUSCULAR administration. Typically these
severe reactions have resembled hypersensitivity or anaphylaxis, including shock and cardiac
and/or respiratory arrest. Some patients have exhibited these severe reactions on receiving
AquaMEPHYTON for the first time.
Therefore, the INTRAVENOUS and INTRAMUSCULAR routes should be restricted to situations where the subcutaneous route is not feasible and the serious risk involved is considered justified.
When intravenous administration is unavoidable, inject very slowly, not exceeding 1 mg per minute.
The subcutaneous route is preferred whenever possible.
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after INTRAVENOUS and INTRAMUSCULAR injection of phytonadione. Severe reactions have occurred despite dilution to avoid rapid infusion and have been reported with first doses. These reactions have included shock, cardiorespiratory arrest, flushing, diaphoresis, chest pain, tachycardia, cyanosis, weakness, and dyspnea.
Severe reactions have also been reported following INTRAMUSCULAR administration, the route commonly used for newborn prophylaxis. Some patients have exhibited severe reactions upon receiving phytonadione for the first time.
Absolute Contraindications & Critical Warnings
Special Populations Requiring Extreme Caution
Adverse Effects Profile#
Common Adverse Reactions (Reported Frequently)#
- Injection Site Reactions: Pain, tenderness, swelling, hematoma formation at injection site (very common with IM administration in newborns)
- Flushing and Diaphoresis: Transient flushing sensations, profuse sweating
- Dysgeusia: Peculiar or altered taste sensations
- Hypotension: Brief hypotension, particularly with IV administration
- Tachycardia: Rapid pulse, palpitations
- Dizziness: Transient dizziness, lightheadedness
- Dyspnea: Difficulty breathing, shortness of breath
- Cyanosis: Bluish discoloration of skin and mucous membranes
- Weakness: General weakness, malaise
Serious and Life-Threatening Adverse Reactions#
- ⚠ Anaphylaxis and Anaphylactoid Reactions: Fatal anaphylaxis, severe hypersensitivity reactions including shock. Can occur with FIRST dose. Black Box Warning applies.
- Cardiorespiratory Arrest: Cardiac arrest, respiratory arrest during or immediately after injection. Deaths have been documented.
- Severe Hypotension and Shock: Profound hypotension, circulatory collapse, cardiovascular shock
- “Gasping Syndrome” in Neonates: Fatal benzyl alcohol toxicity – CNS depression, metabolic acidosis, gasping respirations, seizures, intracranial hemorrhage, cardiovascular collapse, death. Documented in premature infants.
- Hemolysis in Newborns: Hemolytic anemia, particularly in premature infants and G6PD deficiency
- Hyperbilirubinemia and Kernicterus: Excessive bilirubin in newborns, risk of kernicterus (bilirubin-induced brain damage) especially in premature infants
- Intracranial Hemorrhage: Associated with benzyl alcohol toxicity in neonates
- Seizures: Convulsions, particularly in neonates exposed to benzyl alcohol
- Hepatic and Renal Failure: Organ failure associated with benzyl alcohol toxicity
- Hematologic Abnormalities: Thrombocytopenia, coagulation abnormalities
- Aluminum-Induced Neurotoxicity: CNS toxicity from aluminum accumulation, particularly in premature neonates with immature kidneys. Bone toxicity, developmental effects.
Delayed and Chronic Adverse Reactions#
- Cutaneous Reactions: Erythematous, indurated, pruritic plaques at injection sites. May progress to scleroderma-like lesions that persist for long periods (months to years). Erythema perstans. Delayed-type hypersensitivity reactions. Urticaria. Eczematous reactions. Time of onset: 1 day to 1 year after injection.
- Tissue Necrosis: Rare cases of skin necrosis and tissue damage at injection sites, particularly with repeated injections
- Allergic Sensitization: Development of allergy to phytonadione or components after initial exposure, increasing risk of severe reactions with subsequent doses
- Thrombotic Events: Risk of thromboembolic phenomena when large doses restore clotting in patients who previously required anticoagulation (PE, DVT, stroke, MI)
⚠ CRITICAL SAFETY NOTE: The adverse reactions listed above are documented in FDA-approved prescribing information and post-marketing surveillance. Deaths have occurred with both IV and IM administration, which is why the FDA requires a Black Box Warning. Despite these severe risks, vitamin K injection remains routine in newborns.
The “gasping syndrome” from benzyl alcohol preservative is particularly concerning in the neonatal population, where phytonadione is most commonly administered. The FDA explicitly warns about this toxicity but allows benzyl alcohol in injectable formulations given to newborns within hours of birth, when hepatic and renal detoxification systems are most immature.
Drug Interactions#
Clinical Significance
The most clinically important interaction is with warfarin and coumarin anticoagulants, where phytonadione serves as the specific reversal agent. However, this creates a paradox: restoring vitamin K-dependent clotting factor synthesis may restore the original thrombotic tendency that necessitated anticoagulation, putting patients at risk for stroke, PE, DVT, or MI. This is the paradox of drugs and the vicious cycle of harm they can create. The interaction with broad-spectrum antibiotics causing vitamin K deficiency is a common indication for therapeutic phytonadione use in hospitalized patients.
Active & Inactive Ingredients#
AquaMEPHYTON (Merck) and Vitamin K1 Injection (Pfizer/Hospira) are sterile aqueous colloidal solutions/dispersions with the following composition:
Active Ingredient (Per mL)#
- Phytonadione (Vitamin K1): 2 mg/mL (0.5 mL ampuls) OR 10 mg/mL (1 mL ampuls)
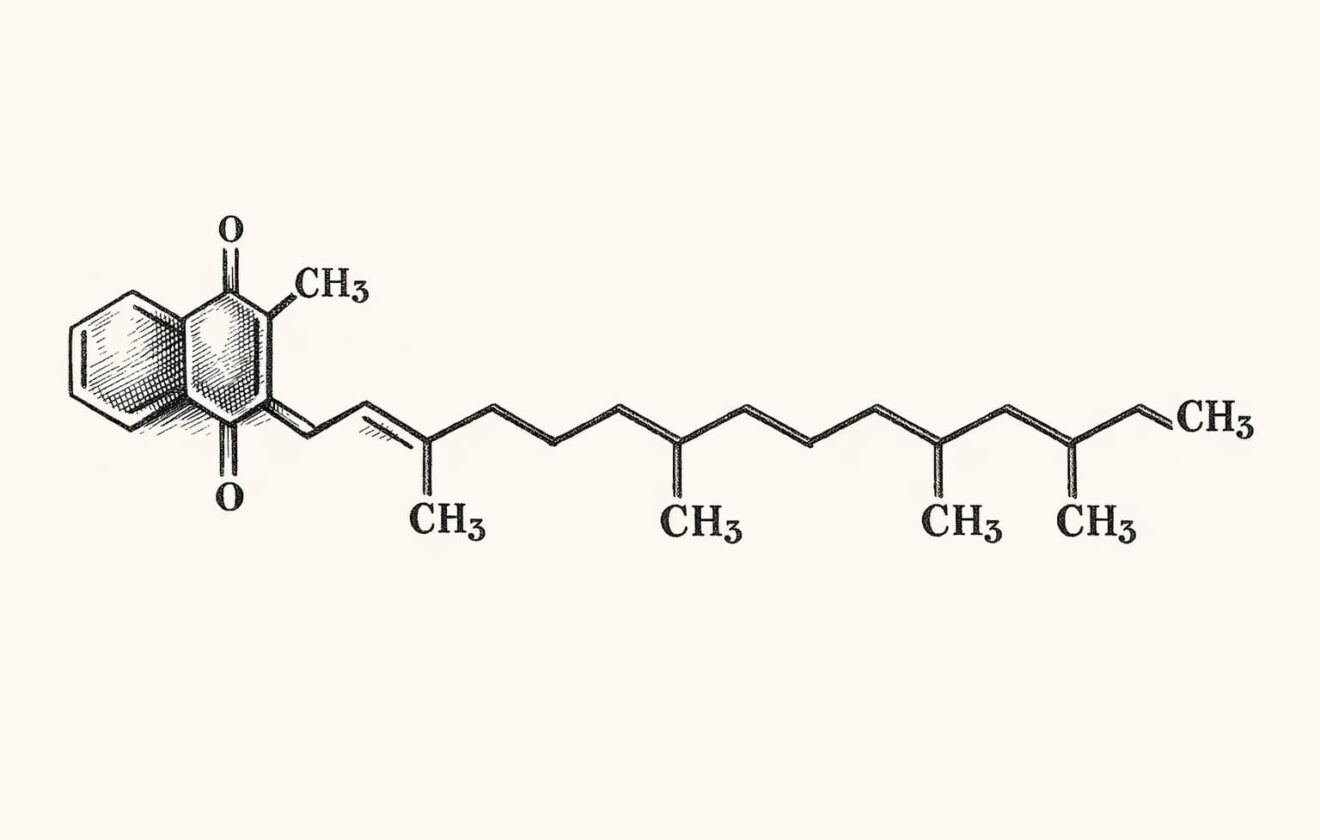
- Chemical Structure: 2-methyl-3-phytyl-1,4-naphthoquinone
- Molecular Formula: C₃₁H₄₆O₂
- Molecular Weight: 450.70 g/mol
- Properties: Clear yellow to amber viscous liquid, odorless, insoluble in water, soluble in chloroform, slightly soluble in ethanol, oxygen sensitive, rapidly degraded by light
⚠ Preservative (Multidose Formulations)#
- Benzyl Alcohol: 9 mg/mL (0.9%) in AquaMEPHYTON and Vitamin K1 Injection multidose vials
- NEUROTOXIC PRESERVATIVE: Associated with fatal “gasping syndrome” in neonates and infants – CNS depression, metabolic acidosis, seizures, intracranial hemorrhage, cardiovascular collapse, death
- FDA Warning: Serious adverse events and death in premature neonates and low birth weight infants. Benzyl alcohol dosages of 99-234 mg/kg/day produced toxic blood levels
- Premature infants cannot metabolize benzyl alcohol effectively due to immature hepatic and renal systems
- NOT present in single-dose prefilled syringes (preservative-free formulations available but not universally used)
Excipients (Inactive Ingredients)#
- Polyoxyethylated Fatty Acid Derivative: 70 mg/mL (emulsifying agent, creates aqueous dispersion of fat-soluble vitamin K. Can cause histamine release and allergic reactions.)
- Dextrose (Hydrous): 37.5 mg/mL (tonicity agent, carbohydrate source)
- Water for Injection: q.s. to 1 mL (sterile vehicle)
- Hydrochloric Acid: May be added for pH adjustment (creates acidic pH)
- pH: 5.0-7.0 (AquaMEPHYTON), 6.3 (5.0-7.0) (Vitamin K1 Injection Pfizer)
⚠ Aluminum Content (Some Formulations)#
- Aluminum: May be present depending on manufacturing process and glass ampul leaching
- FDA WARNING ON LABEL: “This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired.”
- “Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.”
- “Aluminum accumulation at >4-5 mcg/kg/day causes CNS and bone toxicity. Tissue loading may occur at even lower rates of administration.”
- Aluminum accumulates in brain, bone, and kidney tissue. Neurotoxic and causes skeletal abnormalities in developing infants.
Formulation Types and Availability#
- AquaMEPHYTON (Merck):
- 1 mg/0.5 mL single-dose ampuls (5 ampuls per box)
- 10 mg/1 mL single-dose ampuls (5 ampuls per box)
- Contains benzyl alcohol 0.9% (9 mg/mL)
- Vitamin K1 Injection (Pfizer/Hospira):
- 10 mg/mL single-dose ampuls
- Contains benzyl alcohol 9 mg/mL (0.9%)
- Preservative-Free Formulations: Some manufacturers produce benzyl alcohol-free single-dose preparations, but these are not universally available or used
Critical Storage and Handling Requirements#
- ⚠ PROTECT FROM LIGHT AT ALL TIMES: Vitamin K1 is rapidly degraded by light. Store in closed original carton until use.
- Storage Temperature: 20°C to 25°C (68°F to 77°F), USP Controlled Room Temperature
- Oxygen Sensitive: Minimize air exposure
- If Diluted: Administer immediately after dilution. Discard unused portions of diluted solution and unused ampul contents
- Visual Inspection: Inspect for particulate matter and discoloration before administration (should be clear yellow to amber)
⚠ CRITICAL SAFETY NOTE: The presence of benzyl alcohol preservative (9 mg/mL) in standard newborn vitamin K formulations is particularly concerning given that these injections are administered to neonates within hours of birth. A 0.5 mg dose (standard prophylaxis) delivered in 0.5 mL volume contains 4.5 mg of benzyl alcohol. A 1 mg dose in 1 mL contains 9 mg benzyl alcohol. In a 3 kg newborn, this represents 1.5-3 mg/kg of benzyl alcohol in a single injection given to an infant with completely immature hepatic glucuronidation and renal excretion systems. The FDA explicitly warns that “serious and fatal adverse reactions including gasping syndrome can occur in neonates” with benzyl alcohol-preserved drugs, yet this preservative remains in routine newborn vitamin K formulations. Benzyl alcohol-free formulations exist but are not mandated or universally used. Parents are rarely informed of the preservative content or associated risks when consenting to newborn vitamin K injection.

Simply type the keyword and press enter to begin your search.